In-Depth Toxicological Effects Analysis and E-Manual Section Overview
This section describes the final scientific evaluation component of the PHA process. It involves looking more closely at contaminant-specific information in the context of site exposures.
As part of the exposure pathways evaluation, you identified who might come in contact with environmental contaminants, how these individuals might be exposed, and the amount or concentration to which they might be exposed. Then, for completed and potential exposure pathways, you conducted a screening analysis to compare contaminant concentrations to ATSDR-derived, media-specific comparison values (also called CVs) and, if appropriate, non-ATSDR screening levels. In some cases, you performed an EPCs and Exposure Calculations Evaluation, where you determined the exposure point concentrations (EPCs). You then used the EPCs to calculate site-specific exposure doses or adjusted air concentrations. These dose and adjusted air outputs were then used to calculate hazard quotients (HQs) and estimate cancer risks (CRs).
Health assessors perform an in-depth toxicological effects analysis if the potential for harmful health effects was not ruled out based on the evaluation of EPCs and exposure calculations, or for other reasons such as the contaminant is a community concern.
By now, you would have ruled out those exposure pathways and contaminants that pose no health hazards and retained those requiring more examination. You should proceed with performing an in-depth analysis if any of the following conditions are met for your site-specific scenarios:
- A HQ exceeds 1.
- A CR exceeds 1E-6 (i.e., cancer risk exceeds one extra case in a million people similarly exposed).
A more in-depth review of the health effects data might also be necessary in other cases. Examples include the following:
- No health guideline is available to evaluate non-cancer health effects for a contaminant. (Exceptions can include essential nutrients and other constituents naturally found in environmental media, such as calcium, potassium, and magnesium).
- No oral cancer slope factor (CSF) or inhalation unit risk (IUR) is available to calculate cancer risk for a known or suspected carcinogen.
- A contaminant is a community concern.
- Other factors (such as concerns about specific sensitive populations, multiple pathways of exposure, or mixture effects) warrant further evaluation.
So far in the public health assessment (PHA) process, you know the numeric value of the health-based guideline used to assess non-cancer health effects (e.g., Minimal Risk Levels [MRLs], Reference Doses [RfDs], Reference Concentrations [RfCs]) and the cancer risk value used to assess cancer effects (e.g., oral cancer slope factors [CSFs] and inhalation unit risks [IURs]). Health effects and toxicological information are necessary to develop these health guidelines and cancer risk values, but up to this point, you haven’t directly used this type of information about the contaminants of interest. Now, to investigate whether non-cancer or cancer effects are possible because of site exposures, you will look more closely at contaminant-specific information. This analysis will provide perspective on what it means when an HQ of 1 or a CR of 1E-6 has been exceeded, and in some cases, how to address site-specific factors that require further evaluation.
During this analysis, you will perform certain steps as covered in this section to examine whether health effects could be possible as a result of site-related exposure. Of the steps covered in this section, those you need to take will vary depending on certain factors, such as whether you are examining non-cancer or cancer effects and if there is a non-cancer health guideline available. You can perform the steps in the order of your preference if you take the steps appropriate for your site-specific evaluation. The figure below can help you follow the overall process and decision logic for the in-depth toxicological effects analysis.
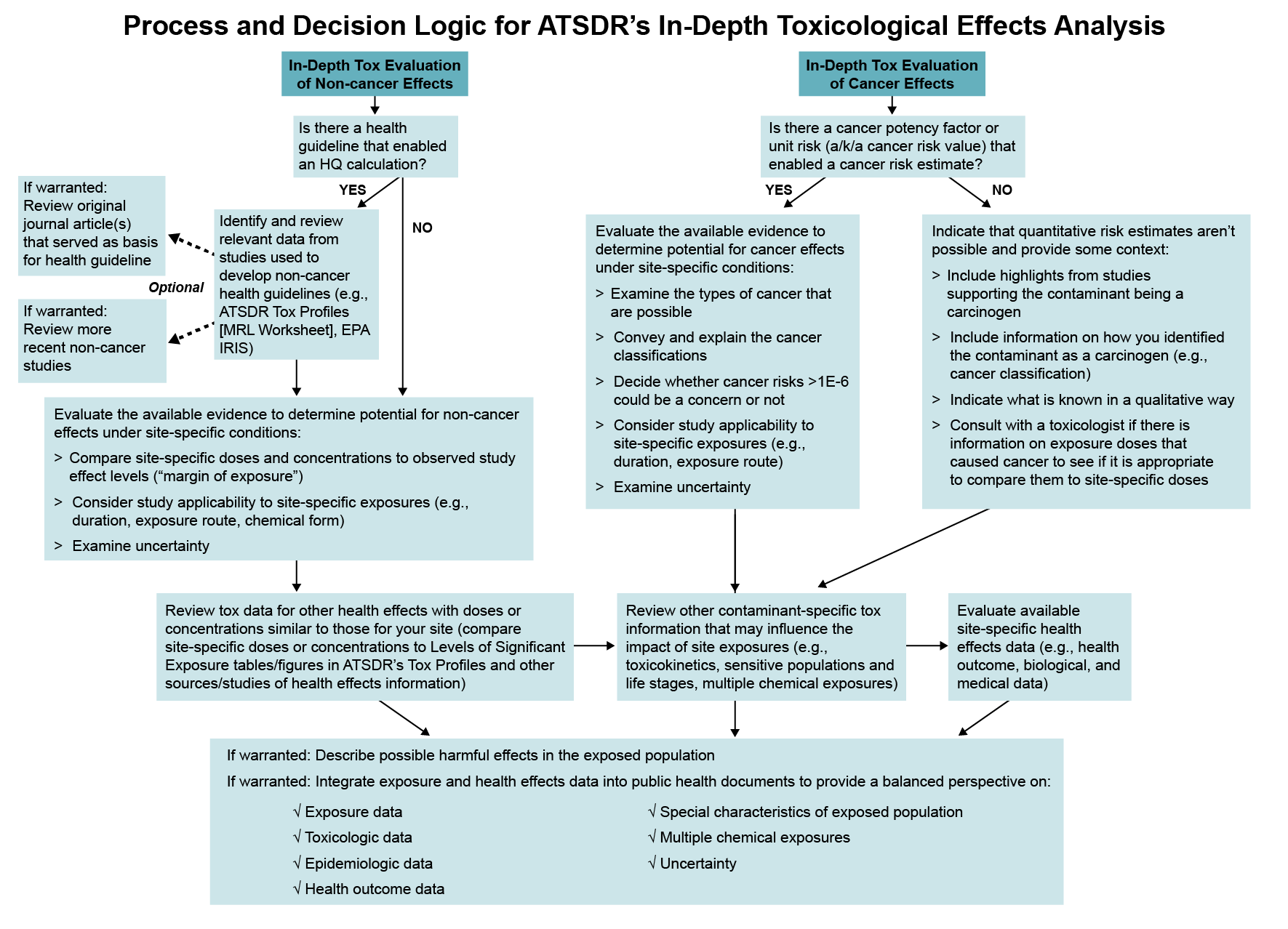
During the in-depth toxicological analysis, you will review information to understand questions such as these:
- How does the contaminant get into the body?
- What happens to the contaminant after it gets into the body?
- What data were used to develop the health guidelines and/or cancer risk values?
- What health effects are associated with the contaminant and at what doses or concentrations?
- How do site-specific doses or concentrations compare to health effects doses or concentrations in published studies?
The analysis will then help you find answers about:
- What harmful effects might be expected in exposed people?
- What public health actions are needed to prevent or reduce exposures?
You will evaluate and integrate exposure data (e.g., site-specific exposure conditions, doses, concentrations) and contaminant-specific health effects data from toxicologic or epidemiologic studies. The result of this in-depth toxicologic analysis is a qualitative description of whether site-specific exposures could adversely affect public health. The findings will help ATSDR determine health conclusions and recommendations for public health actions.
When comparing site-specific doses and concentrations to health guidelines and study effect levels, you will almost always find some uncertainty in deciding possible health effects in the exposed population. Therefore, a key goal of the PHA process is to put site-specific exposures and the potential for harm in perspective. The narrative describing your health effects and findings should therefore describe the uncertainties about the exposures, concentration estimates, doses, and toxicity.
But during this process, you may sometimes have insufficient toxicological data to perform any type of meaningful comparisons for your site-specific contaminants and exposure conditions. In these cases, review the site-specific exposure potential and determine whether the limited toxicity data are a critical information gap to assessing the possibility of site-related health effects. If so, the site team might recommend the need for further research. Remember, you need to clearly explain and justify what is known and what is unknown about the toxicity of the contaminant in question.
Use this checklist to guide you through the in-depth evaluation process.
The PHA process will not require you to consider all the elements of the in-depth analysis described in this section for all sites. The level of analysis will differ across sites and will depend on the scope and complexity of site-related issues, such as the magnitude of exposures, the contaminants under evaluation, and specific community health concerns.
As you review and integrate exposure and health effects information, use your professional judgment to weigh what is known and unknown, including uncertainties and data limitations. You might need assistance from team members or other technical specialists in toxicology, epidemiology, medicine, and health physics. As the health assessor, you will be responsible for integrating and communicating the findings of this analysis into your documents.
Remember: One of the primary goals of ATSDR’s PHA process is to provide reliable, understandable information for the public. The in-depth evaluation is designed to guide the health assessor in conducting the detailed analysis needed to communicate that environmental and health information.
Resources Needed to Support the In-Depth Analysis
There are several sources you will commonly use during the in-depth analysis (see the table below). When identifying the most relevant and up-to-date sources of data to support your analysis, you might need to consult with appropriate subject matter experts. Conducting a critical review of toxicologic or epidemiologic data requires specialized training and a thorough understanding of underlying scientific principles. Similarly, a health physicist will need to assist in identifying appropriate resources for evaluating radiological hazards, or if possible, evaluate the radiological hazards for you. If available resources (such as Toxicological Profiles) have not been recently updated, you must identify the current state of the knowledge for a particular contaminant. Though ATSDR continually reviews contaminant-specific toxicologic data, some of the profiles could be outdated. To determine the status of any ongoing contaminant-specific research, reach out to ATSDR’s toxicologists and other experienced scientists, including chemical managers who work on either developing the Toxicological Profiles or evaluating Superfund sites.
Common Data Sources
Data Source |
Types of Information |
|---|---|
| Toxicological Information Resources | |
| ATSDR’s Toxic Substances Portal | A variety of information on toxic substances, such as toxicological information by chemical, health effect, chemical class, or audience. This portal serves as a gateway to a vast number of ATSDR resources, such as Toxicological Profiles and ToxGuides. |
| ATSDR’s Toxicological Profiles | Contain information on more than 200 contaminants commonly found at hazardous waste sites. ATSDR updates the Toxicological Profiles periodically. Recent profiles should provide all the information you need to support your analysis and draw public health conclusions. Each peer-reviewed profile contains the following:
Consult with the profile’s chemical manager to determine the status of any ongoing research, especially for contaminants with profiles that have not been recently updated. |
| ATSDR’s ToxGuides | Developed as quick reference sheets for health assessors. They provide information for numerous contaminants covering various topics such as on chemical and physical properties, sources of exposure, routes of exposure, and health effects. |
| ATSDR’s Public Health Statements (PHSs) | Available in some Toxicological Profiles as summary stand-alone resources, which are pulled from Chapter One of each contaminant-specific Toxicological Profile. |
| ATSDR’s ToxFAQs | Fact sheets for the public that summarize the contaminant-specific information in the Toxicological Profile to help guide understanding of the contaminant-specific effects of exposure on human health. |
| ATSDR’s Interaction Profiles | Characterize the toxicologic and adverse health effects information for certain defined contaminant mixtures. These profiles help health assessors evaluate data on the toxicology of the whole priority mixture (if available) and on the joint toxic action of the chemicals in the mixture. Each peer-reviewed profile reviews the key literature that describes toxicologic properties of the featured mixture. |
| ATSDR’s Minimal Risk Levels (MRLs) | Lists ATSDR’s available MRLs, which are estimates of the daily human exposure to a contaminant that is likely to be without appreciable risk of harmful non-cancer health effects over a specified duration of oral or inhalation exposure. |
| CVs and Health Guidelines Module (in ATSDR’s Public Health Assessment Site Tool [PHAST]) | Provides information to assist with screening contaminants and with calculating exposure doses, HQs, and CRs. This module also maintains toxicological information on contaminants housed in PHAST. ATSDR and its state-partner health assessors, as well as others performing work related to the PHA process, can access PHAST by emailing phast@cdc.gov. |
| ATSDR’s Support Document to the Substance Priority List | Describes the methodology ATSDR uses to generate its Substance Priority List. This list includes contaminants to be of greatest public health concern to persons at or near National Priority List (NPL) sites. Each contaminant on the list is a candidate to become the subject of a Toxicological Profile prepared by ATSDR. |
| EPA’s Integrated Risk Information System (IRIS) Database | Includes summaries and toxicological reviews that provide the basis of EPA’s health guidelines (RfDs and RfCs) and cancer risk values (CSFs and IURs). EPA also classifies chemicals according to their carcinogenicity, and those classifications are included in PHAST. |
| International Agency for Research on Cancer (IARC) Monographs | IARC is the cancer agency within the World Health Organization (WHO). These scientific documents evaluate the carcinogenic potential of contaminants to humans and serve as the basis for IARC cancer classifications that are included in PHAST. |
| National Library of Medicine (NLM) | Houses chemical and toxicological information on numerous contaminants. Chemical information is available in NLM’s PubChem and ChemIDplus databases. Toxicological literature is available via PubMed. |
| National Toxicology Program (NTP) | NTP is a division of the National Institute of Environmental Health Sciences. To predict whether a contaminant will cause harm to humans, NTP develops and carries out comprehensive toxicological studies, and publishes study findings in cancer and non-cancer health assessment reports. NTP also classifies chemicals according to their carcinogenicity, with those classifications included in PHAST. |
| Peer-reviewed scientific journals | These journals cover topics of environmental toxicology and environmental health. Several journals are dedicated to radiation protection, radiation biology, and the radiological sciences and environment. |
| Standard toxicology textbooks | Information is available in toxicology textbooks (e.g., Casarett & Doull’s Toxicology) for more in-depth evaluations, or in the absence of other sources. |
| The Risk Assessment Information System (RAIS) | Provides information on chemical toxicity and chemical data profiles. |
| Radiation Information Sources | |
| ATSDR radiation guidance documents | Refer to the Basic Introduction to Dose Determination from Radioactive Materials (July 1, 2016) for general information on estimating doses for exposures to radioactive materials. Other documents are in development, on topics such as radiation statistics and the importance of background levels of radioactive materials in assessing environmental samples. |
| International Commission on Radiological Protection (ICRP) | An international organization that advances the science of radiation protection through the science of radiological dose determinations by scientific consensus. The ICRP recommendations are disseminated by publications and its journal, Annals of the ICRP, on its website. |
| National Council on Radiation Protection and Measurements (NCRP) | A U.S. government-chartered organization that formulates guidance and recommendations for radiation protection and measurements using a consensus process. The NCRP releases its determinations as commentaries and publications on its website. |