Process and Decision Logic for ATSDR’s In-Depth Toxicological Effects Analysis
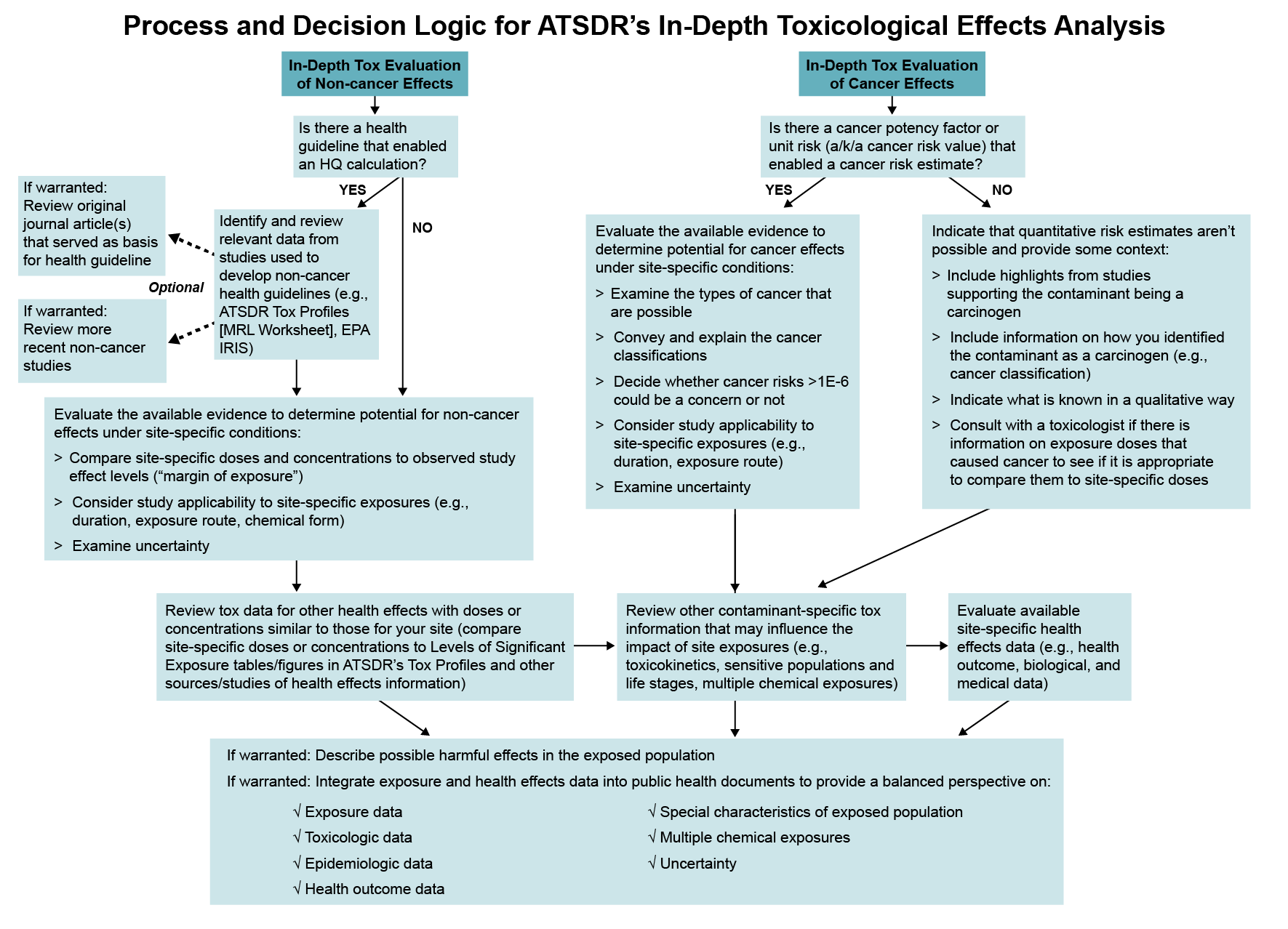
This diagram shows the process and decision logic for a health assessor to follow during the In-Depth Toxicological Effects Analysis. For the In-Depth Tox Evaluation of Non-Cancer Effects, the first box asks, “Is there a health guideline that enabled an HQ calculation?” If the answer is yes, an arrow goes to a box that says: “Identify and review relevant data from studies used to develop non-cancer health guidelines (e.g., ATSDR Tox Profiles [MRL Worksheet], EPA IRIS.” There are then arrows pointing to two optional steps: “If warranted: Review original journal article(s) that served as basis for health guideline” and “If warranted: Review more recent non-cancer studies.” For an answer of yes or no to whether a health guideline enabled an HQ calculation, arrows go to the next box that says: “Evaluate the available evidence to determine potential for non-cancer effects under site-specific conditions that includes bullets:
- Compare site-specific doses and concentrations to observed study effect levels (“margin of exposure”)
- Consider study applicability to site-specific exposures (e.g., duration, exposure route, chemical form)
- Examine uncertainty”
If the answer is no, an arrow goes to a box that is also under non-cancer that says: “Review tox data for other health effects with doses or concentrations similar to those for your site (compare site-specific doses or concentrations to Levels of Significant Exposure tables/figures in ATSDR’s Tox Profiles and other sources/studies of health effects information).”
For the In-Depth Tox Evaluation of Cancer Effects, the first box asks, “Is there a cancer potency factor or unit risk (a/k/a cancer risk value) that enabled a cancer risk estimate?” If the answer is yes, an arrow goes to a box that says: “Evaluate the available evidence to determine potential for cancer effects under site-specific conditions that includes these bullets:
- Examine the types of cancer that are possible
- Convey and explain the cancer classifications
- Decide whether cancer risks >1E-6 could be a concern or not.
- Consider study applicability to site-specific exposures (e.g., duration, exposure route)
- Examine uncertainty”
If the answer is no, an arrows goes to a box that says: “Indicate that quantitative risk estimates aren’t possible and provide some context that includes these bullets:
- Include highlights from studies supporting the contaminant being a carcinogen
- Include information on how you identified the contaminant as a carcinogen (e.g., cancer classification)
- Indicate what is known in a qualitative way
- Consult with a toxicologist if there is information on exposure doses that caused cancer to see if it is appropriate to compare them to site-specific doses”
For both the non-cancer and cancer effects sides, the next arrows both go to a box that says: “Review other contaminant-specific tox information that may influence the impact of site exposures (e.g., toxicokinetics, sensitive populations and life stages, multiple chemical exposures)” and then to a box that says: “Evaluate available site-specific health effects data (e.g., health outcome, biological, and medical data).” The last box connected to non-cancer and cancer evaluations says: “If warranted: Describe possible harmful effects in the exposed population” and “If warranted: Integrate exposure and health effects data into public health documents to provide a balanced perspective on these bulleted components:
- Exposure data
- Toxicologic data
- Epidemiologic data
- Health outcome data
- Special characteristics of exposed population
- Multiple chemical exposures
- Uncertainty”