What to know
This clinician brief describes properties and sources of arsenic, routes of exposure, populations at risk, health effects, and clinical evaluation, management, and counseling of patients.

Properties
Arsenic is a naturally occurring element that is widely distributed in the Earth's crust. It is found in soil, mineral ores, groundwater, volcanic eruptions, and seafood. It is also used in many industrial processes, including metal smelting, wood preservation, and arsenical pesticide production and application. Arsenic is usually found in the environment combined with other elements and is commonly classified as inorganic or organic arsenic. Most inorganic and organic arsenic compounds are white or colorless powders that do not evaporate. They have no smell, and most have no particular taste. Inorganic arsenic compounds are highly toxic and contain non-carbon elements such as oxygen, chlorine, and sulfur. Organic arsenic compounds contain carbon and are generally less toxic than inorganic arsenic [ATSDR 2007].
Sources
Arsenic is found in a wide range of products and materials. Because arsenic is a natural component of the Earth's crust, it exists in the air, water, and soil. Both human and natural activity can release arsenic into the environment. Human activities that release arsenic into the environment include mining, metal smelting, and other industrial operations [ATSDR 2007].
Inorganic arsenic is found in
- Crops such as rice, corn, fruits, and vegetables that are grown in contaminated soil or irrigated with contaminated water [Arslan et al. 2017]
- Meat from animals that consumed crops or water containing inorganic arsenic
- Industrial metal alloys and agents involved in the processing of glass, pigments, textiles, paper, metal adhesives, wood preservatives, and ammunition
- Agents for hide tanning
- Some traditional, imported, homeopathic, and naturopathic remedies
Inorganic arsenic-based pesticides were once widely used for a variety of agricultural and home applications. However, they were discontinued for most uses in the United States during the 1980s and 1990s [IARC 2012]. Today there are limited permitted uses of pesticides containing monosodium methanearsonate (MSMA), an organic arsenical that is converted into inorganic arsenic in the environment [EPA 2006].
Seafood naturally contains arsenobetaine and arsenocholine, which are organic forms of arsenic that are essentially nontoxic [ATSDR 2007].
Routes of Exposure
Ingestion
Ingestion of food containing arsenic is the main source of exposure for the general U.S. population. Intake from air, soil, and drinking water usually contributes much less to human exposures in the United States. Food can contain organic and inorganic forms of arsenic. Seaweed, rice, fruits, meat, shellfish, and poultry are potential dietary sources of inorganic arsenic. Seafood, particularly finfish, contains mostly nontoxic organic arsenic and is unlikely to be a cause of arsenic toxicity [Arslan et al. 2017; ATSDR 2007].
Plants can accumulate arsenic by root uptake from the soil or by absorption of airborne arsenic deposited on the leaves. Certain plant species may accumulate substantial levels [ATSDR 2007]. Rice tends to have higher arsenic concentrations than other cereal crops, such as wheat and barley. This is due to rice's ability to take up arsenic from soil and water. Rice is also typically grown under flooded conditions, which increases the potential for arsenic uptake [FDA 2020]. Other plant species take up comparatively low levels of arsenic, even when grown in highly polluted soil or soil naturally high in arsenic.
Elevated arsenic levels in soil due to contamination may pose an ingestion risk for children with mouthing behaviors during play. Chromated copper arsenate (CCA) is a wood preservative that was previously used in residential structures such as playgrounds. Arsenic can leach from CCA-treated lumber into soil, and children with soil-pica behavior (the intentional ingestion of soil) are at particular risk for arsenic exposure. Because of this risk, in 2003 the U.S. Environmental Protection Agency (EPA) and the lumber industry agreed to discontinue the use of CCA-treated wood in most residential construction. The chemical form of arsenic in soil is an important variable that affects the bioavailability of arsenic and how much is absorbed through the gut [ATSDR 2007].
Groundwater can also contain elevated concentrations of arsenic from agricultural and home pesticide runoff, mining activities, improperly disposed arsenical chemicals, and dust from coal burning [ATSDR 2007].
Well water contaminated by arsenic from natural sources such as bedrock has been identified as a cause of arsenic toxicity worldwide. Countries where arsenic toxicity has been reported from natural source contamination of well water include the United States, Argentina, Bangladesh, China, Germany, India, and Japan [Argos et al. 2012]. Areas in the United States with the highest natural groundwater arsenic concentrations are the Southwest, Northwest, Northeast, Alaska, and areas near geothermal activity [ATSDR 2007]. U.S. residents with private wells might be unaware of their potential risk for arsenic exposure.
The EPA sets drinking water standards to control the level of contaminants in the nation's drinking water in municipal water supplies. The EPA has established a drinking water standard of 10 micrograms of arsenic per liter of water [EPA 2001]. This standard is based on total arsenic, but drinking water contains almost entirely inorganic forms [EPA 2002].
Inhalation
Inhalation of particulate matter from burning arsenic-containing wood or coal can be a significant route of inhalational exposure. Larger particles are deposited in the upper airways and swallowed after coughing, resulting in gastrointestinal absorption. Smaller particles are deposited more deeply in the respiratory tract, where absorption through lungs can occur. Sources of inhaled arsenic for the public include tobacco smoke, burning of CCA-treated wood or arsenic-containing coal, and emissions from nearby smelting operations [ATSDR 2007].
Dermal
Arsenic has poor dermal absorption. Contact with preserved wood products containing arsenic could conceivably result in arsenic exposure, but not enough information is known to make statements about skin absorption in specific conditions [ATSDR 2007].
Populations at Risk
Individual and population differences in nutritional status and the ability to metabolize arsenic could affect susceptibility to arsenic toxicity [Argos et al. 2012; ATSDR 2007, 2016]. Specifically, there is some evidence that low dietary protein intake and possibly other nutritional deficiencies, such as vitamin B-12 deficiency, can decrease arsenic methylation. Arsenic methylation is the main metabolic route by which the body detoxifies arsenic. Impairment of this pathway can increase susceptibility to arsenic toxicity.
In addition to populations with poor nutritional status, other populations at higher risk of exposure to arsenic include people who
- Obtain water from arsenic-contaminated wells
- Live in areas with highly contaminated soils
- Burn coal or pressure-treated wood for home heating
- Engage in hobby activities that could involve the use of arsenic-containing products, such as gardening with arsenic-containing pesticides
Furthermore, people whose jobs involve the use or production of arsenic are at higher risk of exposure [ATSDR 2007, 2016], including
- Miners
- Copper and lead smelters
- Agricultural workers applying arsenic-containing pesticides
- Wood treatment workers who apply CCA to lumber
- Workers in microelectronics or semiconductor industries
Pediatric Populations
Children are uniquely susceptible to toxic exposures. In general, children breathe more air, drink more water, and eat more food per pound of body weight than adults do. They are also more likely to put their hands in their mouths. A child's developing organs and systems also might not be able metabolize and excrete harmful contaminants that enter their bodies. Additionally, children have more time to develop health conditions and diseases than people who are exposed later in life. Health problems from an environmental exposure can take years to become evident.
Fetal development is another period of vulnerability to toxic exposures. Exposure to hazardous substances in utero can potentially affect the development of fetal organs and systems. Such exposures can further lead to gross structural changes or more subtle functional changes. Arsenic is known to cross the placenta, and the levels of arsenic in cord blood closely approximate those in maternal blood [ATSDR 2007].
There is growing evidence from human and animal studies that exposure to inorganic arsenic during prenatal development could increase the risk of adverse health effects. These adverse effects include impaired development in utero and neurodevelopmental toxicity in infants and young children [ATSDR 2016]. A literature review by Tolins et al. [2014] highlighted animal model evidence that links prenatal arsenic exposure to reduction in brain weight, fewer neurons, and alterations in neurotransmitter systems. The same review compiled epidemiological studies in humans demonstrating that early life exposure to arsenic is associated with deficits in intelligence and memory.
Breast milk can contain low levels of arsenic. Exposure can also occur when contaminated water is used to make formula or given to children to drink. Additional research on maternal kinetics and transfer via breast milk could provide a more complete picture of prenatal and neonatal development, including neural development and the possible development of childhood cancer [ATSDR 2007, 2016]. However, breast milk continues to be the ideal nutrition for infants, despite the potential presence of environmental contaminants. In nearly every circumstance, CDC and the American Academy of Pediatrics recommend that nursing individuals continue to breastfeed. Clinicians can help patients decide to breastfeed based on factors specific to the patient and the child.
Infants and children are more susceptible than adults to toxicity from ingestion of inorganic arsenic. They have less varied diet patterns and consume more food relative to their body weight. Therefore, elevated levels of inorganic arsenic in foods that infants eat could represent a significant source of exposure [FDA 2020].
The Food and Drug Administration (FDA) aims to reduce health effects associated with exposure to inorganic arsenic early in life. Rice cereals are the most consumed infant instant cereals in the United States. The FDA has determined an action level of 100 micrograms per kilogram (μg/kg) or 100 parts per billion (ppb) for inorganic arsenic in infant rice cereals [FDA 2020].
Health Effects
The health effects of arsenic depend on the following:
- Form of arsenic
- Frequency of exposure
- Duration of exposure
- Dose of exposure
- Route of exposure
- Susceptibility of the patient, including genetic factors and nutritional status
Acute Exposure
Acute toxicity from arsenic infrequently results from an intentional or accidental ingestion of a large amount of inorganic arsenic. It is difficult to determine the fatal dose in humans because there are only case reports of acute exposure in the literature [ATSDR 2007].
The classic presenting symptoms of acute arsenic toxicity include
- Severe hemorrhagic gastroenteritis with abdominal pain, vomiting, and "bloody rice water" diarrhea
- Dehydration with hypotension and cardiovascular collapse
- Multisystem organ dysfunction with elevated liver enzymes, renal injury, and disseminated intravascular coagulation
Acute tubular necrosis with acute renal failure has occurred with acute arsenic exposure.Encephalopathy has been reported within 24 to 72 hours following acute exposure, as well as in chronic exposures. Milder clinical features associated with acute exposure include numbness, muscle cramps, facial edema, and gastrointestinal effects such as nausea, vomiting, and diarrhea [ATSDR 2007].
Chronic Exposure
Chronic toxicity can occur when an individual is exposed to low levels of arsenic over months to years, usually from contaminated drinking water such as well water. Skin lesions are among the most common and characteristic effects of arsenic ingestion in humans [ATSDR 2016]. Initial symptoms of long-term exposure to high levels of inorganic arsenic include pigmentation changes and keratosis. Hyperpigmentation is characterized as raindrop-like spots of pigmentation, diffuse dark brown spots, or diffuse darkening of the skin on the limbs or trunk. Areas of hyperpigmentation can be interspersed with small areas of hypopigmentation on the face, neck, and back. Keratosis can present as diffuse thickening of the skin or as nodules on the palms or soles. These skin lesions develop after a minimum exposure of approximately five years and can be a precursor to nonmelanoma skin cancer [WHO 2022].
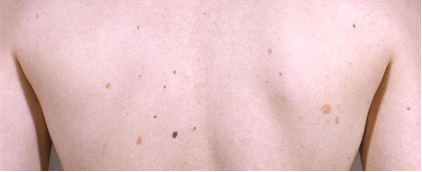
Image 1. A patient with numerous cutaneous arsenical keratoses following chronic arsenic exposure [McLeod & Craig 1965].
Peripheral neuropathy can also occur and could be one of the only symptoms of chronic arsenic exposure. This neuropathy occurs in a symmetrical stocking-glove distribution in the hands and feet. It results from damage to the sensory neurons more than the motor neurons [ATSDR 2007, 2016].
Other adverse health effects can be associated with long-term ingestion of inorganic arsenic. They include developmental effects, pulmonary disease, type 2 diabetes mellitus, and cardiovascular disease. Hematological effects can include bone marrow suppression and pancytopenia. Inorganic arsenic can also affect the female and male reproductive systems. Females can have decreased weight of the ovaries and uterus, while males can have reduced weight of the testes and accessory sex organs, as well as decreased epididymal sperm counts [ATSDR 2007, 2016].
The EPA, the U.S. Department of Health and Human Service's National Toxicology Program, and the International Agency for Research in Cancer (IARC) have classified arsenic and arsenic compounds as carcinogenic to humans [IARC 2012; IRIS 2002; NTP 2023]. Arsenic exposure is associated with lung cancer, nonmelanoma skin cancers, and bladder cancer. There is limited evidence for association with kidney, liver, and prostate cancers [ATSDR 2007, 2016; IARC 2012].
Since arsenic-associated diseases have long latency periods–often several years for skin changes and several decades for cancers–most patients remain asymptomatic for years despite ongoing exposure [ATSDR 2007].
Clinical Evaluation
Patient History
When evaluating a patient potentially exposed to arsenic, it is important to start with a detailed exposure history. Assess possible current and past environmental exposures and ask about the patient's current and previous occupations. Perform a physical exam that pays close attention to skin and nervous system findings and laboratory evaluation when indicated in chronic exposure.
The ATSDR Case Studies in Environmental Medicine: Taking an Exposure History provides downloadable history forms and is a valuable resource in collecting exposure histories [ATSDR 2015].
When asking about environmental exposure history, assess where the patient spends a significant amount of time. Focus on proximity to mining sites, orchards, farms, CCA-treated wood playground structures, and hazardous waste sites. It is also important to ask about arsenic-contaminated sources of water for drinking, cooking, and making infant formula, especially private well water sources [ATSDR 2007, 2015].
Other environmental exposure history questions should focus on a patient's hobbies. Ask about current and historical use of arsenic-based pesticides for gardening inside and outside the home. Ask the patient about home heating methods, particularly CCA-treated wood- or coal-burning stoves, fireplaces, and other sources of fuel. When taking a medication history, ask about traditional, imported, homeopathic, and naturopathic remedies that can contain arsenic.
Questions about occupational history should focus on work in industries that could be at risk for arsenic exposure, such as the manufacturing of microelectronics or semiconductors and pesticide application [ATSDR 2007].
Physical Examination
The physical examination should focus on major organs and systems that arsenic could affect, including the skin and the nervous system. Specifically, check for evidence of skin lesions and peripheral neuropathy [ATSDR 2007].
Diagnostic Tests
The symptoms of arsenic toxicity can be nonspecific or delayed, which can make an early clinical diagnosis of arsenic toxicity difficult.
Urinary arsenic tests can be ordered as total or speciated arsenic. The most helpful laboratory test for recent arsenic exposure is a 24-hour urinary speciated arsenic level, which can distinguish toxic inorganic arsenic from nontoxic organic arsenic. However, total arsenic is often the only 24-hour urine test available. A total urinary arsenic level includes inorganic arsenic and its metabolites that may result in toxicity. It also includes nontoxic organic arsenic, such as arsenobetaine and arsenocholine, from seafood consumption within the past 48 hours.
Spot urine specimens for arsenic and creatinine can be helpful in an emergency [ATSDR 2007].
Although tests of nails and hair for arsenic can indicate chronic toxicity, they are of limited clinical utility because there are no accepted reference ranges or population means [ATSDR 2007].
In exposed patients, a complete blood count can evaluate for hematological effects including anemia, leukopenia, and thrombocytopenia. Blood glucose testing can assess for hyperglycemia [ATSDR 2007].
Treatment and Patient Management
Acute and chronic arsenic exposure present with a wide spectrum of signs and symptoms. The symptoms are largely dependent on the route of exposure, chemical form, dose, and time elapsed since exposure [ATSDR 2007].
Laboratory confirmation of arsenic exposure is often not available in time to guide therapy in the acute setting. Therefore, treatment must often be initiated based on history and clinical findings alone.
Acute Exposure
Patients with suspected acute arsenic poisoning generally require aggressive supportive care and management in an intensive care setting. Care and management options include gastrointestinal decontamination and hemodynamic stabilization with fluid and electrolyte replacement.
Chelating agents, such as dimercaprol, can prevent the effects of arsenic toxicity if administered within a few hours of arsenic exposure [ATSDR 2007]. However, all chelating agents can have potentially life-threatening side effects. When considering chelation therapy, consult with a medical toxicologist or other specialist with expertise and experience in managing acute toxicity. Poison control centers have medical toxicologists available for consultation.
Chronic Exposure
In cases of chronic arsenic toxicity, the primary goal is to end the exposure by first identifying and then mitigating or preventing further exposure to arsenic. Removal of the toxic arsenic source might not always be possible or feasible. Symptoms generally improve following cessation of exposure but can persist with an extended recovery period. Treatment depends on several factors, including urinary arsenic levels, duration of exposure, severity of neurological damage, and current symptoms. Manage symptomatic patients with supportive care [ATSDR 2007].
Patient Follow Up
Periodic clinical evaluations of patients exposed to arsenic can detect abnormalities at an early stage. Further testing can be based on symptoms, physical exam findings, and standard clinical practice. Refer to screening recommendations for cancer and other chronic diseases from the U.S. Preventive Services Task Force (USPSTF). Consider consulting a specialist in medical toxicology or occupational and environmental medicine to develop a plan for periodic monitoring if needed. The Pediatric Environmental Health Specialty Units (PEHSUs) are a national network of experts in the prevention, diagnosis, management, and treatment of health issues that arise from environmental exposures from preconception through adolescence. PEHSUs can provide evidence-based information about arsenic exposure that affects children and families.
Patient Counseling and Risk Reduction
Avoid exposure to toxic arsenic whenever possible. Because the main route of exposure to arsenic is ingestion, encourage patients to do the following:
- Confirm that the arsenic level in their drinking water is below the EPA drinking water standard of 10 micrograms per liter [EPA 2001]. State and local health departments can provide information on arsenic levels in drinking water.
- Have their well water tested through a local health department to ensure arsenic concentrations are below the EPA drinking water standard.
- Remove arsenic in drinking water with certified filters. If removal methods are not feasible, use an alternative source of water for drinking, food preparation, cooking, brushing teeth, and other activities that could result in ingestion of water.
- Do not worry about showering and bathing in household water. Arsenic in household water does not volatilize nor does it readily absorb through the skin.
- Use a safe water supply for gardening and crop irrigation.
- Eat a wide range of foods, including a variety of first foods for infants.
- Use personal protective equipment properly if they work with arsenic. Remove their shoes and clothes before entering their homes to avoid exposing family members to arsenic.
Contact Us
For more information about these products, email ATSDR's Environmental Medicine and Health Systems Intervention Section at envmed@cdc.gov.
For questions or information on other products and topics, contact CDC–INFO.
- Argos M, Ahsan H, Graziano JH. 2012. Arsenic and human health: epidemiologic progress and public health implications. Reviews on Environmental Health 27(4):191-5. https://doi.org/10.1515/reveh-2012-0021
- Arslan B, Djamgoz MBA, Akün E. 2017. Arsenic: a review on exposure pathways, accumulation, mobility and transmission into the human food chain. Reviews of Environmental Contamination and Toxicology 243:27–51. https://doi.org/10.1007/398_2016_18
- [ATSDR] Agency for Toxic Substances and Disease Registry. 2007. Toxicological Profile for Arsenic. U.S. Department of Health & Human Services. https://www.atsdr.cdc.gov/ToxProfiles/tp2.pdf
- [ATSDR] Agency for Toxic Substances and Disease Registry. 2015. Case studies in environmental medicine: taking an exposure history. U.S. Department of Health & Human Services. https://www.atsdr.cdc.gov/csem/exphistory/docs/exposure_history.pdf
- [ATSDR] Agency for Toxic Substances and Disease Registry. 2016. Addendum to the arsenic toxicological profile. U.S. Department of Health & Human Services. https://www.atsdr.cdc.gov/toxprofiles/Arsenic_addendum.pdf
- [EPA] U.S. Environmental Protection Agency. 2001. Technical fact sheet: final rule for arsenic in drinking water. U.S. Environmental Protection Agency. https://nepis.epa.gov/Exe/ZyPdf.cgi?Dockey=20001XXC.txt
- [EPA] U.S. Environmental Protection Agency. 2002. Appendix B: arsenic and clarifications to compliance and new source contaminants monitoring; final rule (66 FR 6976). Implementation guidance for the arsenic rule – drinking water regulations for arsenic and clarifications to compliance and new source contaminants monitoring. https://www.epa.gov/sites/default/files/2015-09/documents/2005_11_10_arsenic_ars_final_app_b.pdf
- [EPA] U.S. Environmental Protection Agency. 2006. Revised reregistration eligibility decision for MSMA, DSMA, CAMA, and cacodylic acid. U.S. Environmental Protection Agency. https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/red_G-16_10-Aug-06.pdf
- [FDA] U.S. Food and Drug Administration. 2020. Inorganic arsenic in rice cereals for infants: action level guidance for industry. U.S. Department of Health & Human Services. https://www.fda.gov/media/97234/download?attachment
- [IARC] International Agency for Research on Cancer. 2012. Arsenic and arsenic compounds. In IARC Monographs on the Identification of Carcinogenic Hazards to Humans. World Health Organization. https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono100C.pdf
- [IRIS] Integrated Risk Information System. 2002. Chemical assessment summary: arsenic, inorganic; CASRN 7440-38-2. U.S. Environmental Protection Agency. https://iris.epa.gov/static/pdfs/0278_summary.pdf
- [NTP] National Toxicology Program. 2023. Report on carcinogens, 15 ed. U.S. Department of Health & Human Services. https://ntp.niehs.nih.gov/sites/default/files/ntp/roc/content/profiles/arsenic.pdf
- Tolins M, Ruchirawat M, Landrigan P. 2014. The developmental neurotoxicity of arsenic: cognitive and behavioral consequences of early life exposure. Annals of Global Health 80(4):303-14. https://doi.org/10.1016/j.aogh.2014.09.005
- [WHO] World Health Organization. 2022. Fact sheets: arsenic. United Nations Economic and Social Council. https://www.who.int/news-room/fact-sheets/detail/arsenic
- Image Citation: McLeod WN, Craig B. 1965. ID# 12180 [Photograph]. Public Health Image Library. U.S. Centers for Disease Control and Prevention. https://phil.cdc.gov/Details.aspx?pid=12180

