What Are the Health Effects from Exposure to Nitrates and Nitrites?
Unless conditions exist for reducing nitrate to nitrite in the gut (i.e., high pH and proper intestinal microbial flora), ingested nitrate (NO3-) is metabolized and excreted without producing apparent adverse effects.
- Nitrate in the diet may even enhance host defenses against gastrointestinal pathogens by modulating platelet activity, and possibly even gastrointestinal motility and microcirculation [McKnight et al. 1999; Lundberg et al. 2004; Hill 1999; Lundberg et al. 2008; Webb et al. 2008; Borniquel et al. 2010; Petersson et al. 2007; 2002; Sobko et al. 2006].
- The known toxic effects of nitrate exposure result from the conversion of nitrate to nitrite [Hord 2011; ATSDR 2004].
- The effects of nitrite (NO2-) are the same whether nitrite containing compounds are ingested or inhaled, or nitrite is produced in vivo from nitrate.
Methemoglobinemia is the critical health effect from exposure to nitrates and nitrites. Depending on the percentage of total MetHb, the clinical presentation may be one of oxygen deprivation with cyanosis, cardiac dysrhythmias and circulatory failure, and progressive central nervous system (CNS) effects [Skold et al. 2011]. CNS effects can range from mild dizziness and lethargy to coma and convulsions [Fan and Steinberg 1996; Bradberry 2003; Osterhoudt 2001; Skold et al. 2011]. (See Table 3).
Acute acquired methemoglobinemia is the most important adverse health effect caused by excessive nitrate or nitrite exposure. Methemoglobinemia inducers also work through other mechanisms outside of nitrate and nitrite formation [Nelson and Hostetler 2003; Flomenbaum et al. 2006; Hunter et al. 2011] (See Table 2).
Methemoglobinemia may arise from various etiologies [Harvey et al. 2010; Greer and Shannon 2005; Wright et al. 1999; Nelson and Hostetler 2003]. These etiologies can be grouped into “acquired” and “congenital”. The acquired methemoglobinemias can come from exogenous or endogenous causes.
Exogenous Causes include
- Ingestion, inhalation or dermal exposure to an oxidizing drug or chemical
- Nitrate or nitrite ingestion in water or diet
Endogenous Causes include
- Systemic acidosis as a result of diarrhea and dehydration
- Gastroenteritis without systemic acidosis
Genetic Causes include
- Genetic disorders presenting as cyanosis shortly after birth:
- NADH methemoglobin reductase deficiency (deficiency of enzymes that reduce MetHb back to Hb)
- Type 1- RBC reductase deficiency
- Type 2- Generalized reductase deficiency
- HbM Disease
- NADH methemoglobin reductase deficiency (deficiency of enzymes that reduce MetHb back to Hb)
“Pseudomethemoglobinemias” may occur from misinterpreted co-oximetry results and include sulfhemoglobinemia [Haymond et al. 2005].
Methemoglobin can be formed by directly oxidizing the iron within the hemoglobin molecule or indirectly causing oxidation through the release of free radicals [Lopez-Shirley et al. 1994; Wright et al. 1999; Nelson and Hostetler 2003; Skold et al. 2011].
Methemoglobinemia is a well-recognized hazard of ingestion of nitrates and nitrites [Hord 2011; Knobeloch et al. 2000; Harris et al. 1979; AAP 1970; Kross et al. 1992].
- The first reported case of fatal acquired methemoglobinemia in an infant due to ingestion of nitrate contaminated well water in the United States occurred in 1945 [Comly 1945].
- This condition is also termed “Blue Baby Syndrome”.
- In the following 25 years, about 2,000 similar cases of acquired methemoglobinemia in young infants were reported worldwide; about 10% of such cases resulted in death [Reynolds 2002].
- Sporadic cases and occasional fatalities occurred through the 1980s,1990s and 2000s, most often resulting from ingestion of nitrate-contaminated well water by infants [Fan and Steinberg 1996; Knobeloch et al. 2000; Shearer et al. 1972].
- Methemoglobinemia from ingestion of nitrates involves conversion in the intestinal flora of nitrates to nitrites which are absorbed systemically and act as oxidizing agents [Nelson and Hostetler 2003; Skold et al. 2011].
Hemoglobin molecules contain iron within a porphyrin heme structure.
- The iron in hemoglobin is normally found in the Fe++ state.
- The iron moiety of hemoglobin can be oxidized to the Fe+++ state to form methemoglobin (MetHb).
- Once it is formed, the molecule loses its ability to carry molecular oxygen and reduces its ability to release oxygen to tissues. The increased affinity for bound oxygen results in a left shift of the oxygen-hemoglobin dissociation curve (see Figure 4).
A certain amount of physiologic MetHb formation occurs continuously because red blood cells are bathed in oxygen.
- Several endogenous reduction systems exist to maintain MetHb in the reduced state.
- In normal individuals only about 1% of total hemoglobin is MetHb at any given time [Wright et al. 1999; Jaffe and Hultquist 1995; Skold et al. 2011].
MetHb can be reduced back to hemoglobin by both NADH-dependent and NADPH-dependent (to a lesser degree) MetHb reductase enzymes.
More specifically, the RBC systems responsible for methemoglobin reduction under physiologic conditions include (in order of decreasing methemoglobin reduction):
- NADH methemoglobin reductase (also known as cytochrome b5 methemoglobin reductase, diaphorase I, DPNH-diaphorase, DPNH dehydrogenase I, NADH dehydrogenase, NADH methemoglobin-ferrocyanide reductase)
- Ascorbic acid
- Glutathione
- NADPH methemoglobin reductase (also known as diaphorase II, NADPH dehydrogenase)
[Haymond et al. 2005; McKenzie 2010].
Methemoglobinemia occurs when these systems become overwhelmed, impaired, are lacking or when there is an inherited defect in the structure of the hemoglobin molecule itself (HbM disease) [Nelson and Hostetler 2003; DeBaun et al. 2011; McDonagh et al. 2013].
Two types of inherited enzyme deficiency methemoglobinemia exist. Erythrocyte reductase deficiency occurs when red blood cells lack the enzyme. Generalized reductase deficiency occurs when the enzyme isn’t functional anywhere in the body [DeBaun et al. 2011].
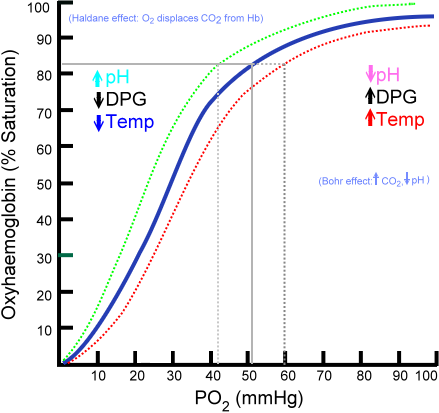
Figure 4. Oxy-Hemoglobin Dissociation Curve. Image Courtesy of Wikimedia Commons viewed in Grethlein SJ and Besa EC. (2012, June 25). Blood Substitutes. Medscape. Retrieved 10/22/13 from http://emedicine.medscape.com/article/207801-overview
Blue line = Normal Hemoglobin
Green line = High affinity Hb, Methemoglobin, “left shift”
Red line = Low affinity Hb, “right shift”
Methemoglobinemia causes a leftward shift in the oxygen-hemoglobin dissociation curve as methemoglobin does not unload O2 from Hb. Sulfhemoglobin causes a right shift in the oxygen-hemoglobin dissociation curve.
Hypotension is the main cardiovascular effect seen with nitrate and nitrite medications and previously thought to be uncommon with ingestion of nitrates and nitrites in food and water.
However, there have been recent studies looking at potential benefits of dietary inorganic nitrates in promoting cardiovascular health [Lundberg et al. 2011; Larsen et al. 2010; Lauer et al. 2008; Sobko et al. 2010; Vanhatalo et al. 2010; Carlstrom et al. 2011; Casey et al. 2007; Webb et al. 2008].
Angina-like pain, MI and cardiovascular death have been reported in explosive manufacturing industry workers exposed to nitroglycerin and other aliphatic nitrates [Hogstedt and Axelson 1977; Hannerz and Greitz 1992; RuDusky 2001].
In the body, nitroglycerin (similar to other nitrites and organic nitrates) is metabolized to nitric oxide (NO) which stimulates a series of events that eventually results in release of calcium ions from smooth muscle cells leading to relaxation and vasodilation.
Increased blood flow in the middle cerebral artery and increased cerebrospinal fluid pressure have been correlated with headache after nitroglycerin exposure [Hannerz and Greitz 1992]. Since the late 1800s, there have been anecdotal reports of explosives workers placing small amounts of explosives in their hatbands when away from work to avoid “powder head” headaches and chest pain on their return to work [Rosenman 2007].
Take home exposures to nitrate dusts on work clothes have been reported to cause headache in exposed family members [Rosenman 2007]. Anecdotal reports of “sudden death’ or “Monday morning angina” leading to death were first described in the 1930s as having been associated with dermal absorption of nitroglycerin and ethylene glycol dinitrate, particularly after being away from work/exposure for a short period of time (i.e. a couple of days/over weekends). Rebound coronary spasm from withdrawal of nitrates is thought to be the underlying mechanism [RuDusky 2001].
Some studies have shown increases in mortality among occupational cohort’s months to years after exposure which would suggest other processes may be involved. Studies and post autopsy reports have supported increased mortality from strokes and heart disease from chronic exposure [Rosenman 2007]. Other studies have not shown increased cardiovascular disease risk from occupational exposure to nitrates [Stayner et al. 1992].
Maternal exposure to environmental nitrates and nitrites may increase the risk of pregnancy complications such as
- Anemia,
- Threatened abortion/premature labor, or
- Preeclampsia [Grant et al. 1995; Tabacova et al. 1997].
Recent epidemiologic data have suggested an association between developmental effects in offspring and the maternal ingestion of nitrate from drinking water such as
- Spontaneous abortions,
- Intrauterine growth restriction, and
- Various birth defects.
However, a definite conclusion on the cause-and-effect relationship cannot be drawn (i.e. in some studies, the potential for confounding could not be determined with certainty due to lack of individual exposure assessment data, etc.) [Manassaram et al. 2006; Fan and Steinberg 1996; Grant et al. 1995; Huber et al. 2013].
The maternal transfer of nitrate, nitrite, and N-nitroso compounds and the potential effect on fetal death and malformation have been described [Bruning-Fann and Kaneene 1993]. Reproductive outcome studies performed at sites with high nitrate levels in the water supply provide some evidence of maternal transfer of nitrate and nitrite [Manassaram et al. 2006; Tabacova et al. 1997 and 1998; Croen et al. 2001].
Further study is needed to determine the relationship between maternal exposure to nitrates and nitrites and reproductive and developmental effects.
Some study results have raised concern about the cancer-causing potential of nitrates and nitrites used as preservatives and color-enhancing agents in meats [Norat et al. 2005; Tricker and Preussmann 1991]. Nitrates can react with amino acids to form nitrosamines, which have been reported to cause cancer in animals [Bruning-Fann and Kaneene 1993]. Elevated risk of non-Hodgkin’s lymphoma [Ward et al. 1996] and cancers of the esophagus, nasopharynx, bladder, colon, prostate and thyroid have been reported [Cantor 1997; Eichholzer and Gutzwiller 1998; Barrett et al. 1998; Ward et al. 2010].
An increased incidence of stomach cancer was observed in one group of workers with occupational exposures to nitrate fertilizer; however, the weight of evidence for gastric cancer causation is mixed [Van Loon et al. 1998; Xu et al. 1992]. Epidemiological investigations and human toxicological studies have not shown an unequivocal relationship between nitrate intake and the risk of cancer [Alexander et al. 2010; Mensinga et al. 2003].
The International Agency for Research on Cancer (IARC) classifies nitrates and nitrites as “probably carcinogenic to humans” (Group 2A) under certain conditions (i.e. ingested nitrate or nitrite under conditions that result in endogenous nitrosation) which could lead to the formation of known carcinogens such as N-nitroso compounds [IARC 2010].
- Acute acquired methemoglobinemia is the most important adverse health effect caused by excessive nitrate/nitrite exposure.
- The known toxic effects from nitrate exposure result from the conversion of nitrate to nitrite.
- The effects of nitrite (NO2-) are the same whether nitrite containing compounds are ingested or inhaled, or nitrite is produced in vivo from nitrate.
- Maternal exposure to environmental nitrates and nitrites may increase the risk of pregnancy complications such as anemia, threatened abortion/premature labor, or preeclampsia.
- Angina-like pain, MI and cardiovascular death have been reported in explosive industry workers exposed to nitroglycerin and other aliphatic nitrates.
- Epidemiological investigations and human toxicological studies have not shown an unequivocal relationship between nitrate intake and the risk of cancer.