What are the Physiologic Effects of Arsenic Exposure?
- Learning Objective
- Introduction
- Historical Exposure Highlighting Multiple Organ System Effects
- Gastrointestinal and Hepatic Effects
- Renal Effects
- Cardiovascular Effects
- Neurologic Effects
- Dermal Effects
- Respiratory Effects
- Hematopoietic and Hematologic Effects
- Reproductive Effects
- Carcinogenic Effects
- Skin Cancer
- Lung Cancer
- Other Health Effects
- Susceptibility to Arsenic Toxicity
- Key Points
- Progress Check
Upon completion of this section, you will be able to
- describe the health effects associated with arsenic exposure.
Because it targets widely dispersed enzyme reactions, arsenic affects nearly all organ systems. The most sensitive endpoint from arsenic exposure is dermal effects. While several studies may identify effects on other endpoints at the same exposure level that produces dermal effects, the database for dermal effects is stronger than for effects on other endpoints [ATSDR 2007]
Key physiologic effects from arsenic exposure that will be covered in detail later.
- Patchy skin hyperpigmentation, small focal keratoses, and other skin lesions are common effects of heavy chronic exposure.
- It is difficult to establish strong associations between arsenic exposure and disease, as the prevalence and spectrum of diseases linked to chronic arsenic exposure differ not only between countries, but even within countries.
- Arsenic can cause lung and skin cancers and may cause other cancers.
- The association between chronic arsenic exposure and cancer is strongest for skin, lung, and bladder cancer. Liver (angiosarcoma), kidney, and other cancers have limited strength of association [IARC 2004; NRC 2000].
Note that when strength of association (defined as the magnitude of the relative risk in the exposed group compared with that in the control group) is mentioned throughout this section, it refers to one of the five criteria used to decide whether a positive association in epidemiologic studies indicates causality. It is not absolutely required to establish causality. In addition, fulfillment of some criteria may occur when the association is a result of chance or bias. Failure to demonstrate a positive association in an epidemiologic study does not always indicate there is no association between the agent and the effects studied.
In the United States, excess cancer mortality associated with arsenic is not generally seen [Schoen et al. 2004].
Unlike other arsenicals, arsine gas causes a hemolytic syndrome.
The description of an historical arsenic exposure highlights its multiple organ system effects.
- Arsenic-contaminated beer resulted in 6,000 poisonings and approximately 71 deaths in Northern England in 1900. Sulfuric acid contaminated with arsenic, which was used to make sugar from sugar cane for use in brewing beer, was found to be the source of the contamination, which affected 100 breweries.
- The common clinical presentation was
- anorexia, brown pigmentation,
- peripheral neuritis (muscular weakness, pain and paresthesias in extremities),
- hepatic lesions,
- localized edema, and
- fatty degeneration of the heart.
- The concentration of arsenic in beer ranged from 2–4 parts per million (ppm) [Reynolds 1901; Aposhian 1989; Engel et al. 1994; Rosenman 2007].
- The common clinical presentation was
The gastrointestinal effects (GI) of arsenic are generally the result of ingestion; however, GI effects may also occur after heavy exposure by other routes.
- GI effects are seen acutely after arsenic ingestion, and less often after inhalation or dermal absorption.
- The fundamental GI lesion appears to be increased permeability of the small blood vessels, leading to fluid loss and hypotension.
- Extensive inflammation and necrosis of the mucosa and submucosa of the stomach and intestine may occur and progress to perforation of the gut wall.
- A hemorrhagic gastroenteritis may develop, with bloody diarrhea as a presenting effect.
Acute arsenic toxicity may be associated with hepatic necrosis and elevated levels of liver enzymes.
- Arsenic intoxication may also result in toxic hepatitis with elevated liver enzyme levels.
- Chronic arsenic ingestion may lead to cirrhotic portal hypertension [ATSDR 2007; Datta 1976].
- There is limited strength of association of chronic arsenic exposure and noncirrhotic portal hypertension [IARC 2004; NRC 2000].
- Case reports have also linked chronic high level arsenic exposure with hepatic angiosarcoma, a rare form of liver cancer [Popper et al. 1978; Zaldivar et al. 1981; ATSDR 2007].
- There is limited strength of association, however, between chronic arsenic exposure and angiosarcoma of the liver, as determined by International Agency for Research on Cancer (IARC) and National Research Council [IARC 2004; NRC 2000].
Arsenic is capable of causing renal effects.
- The systemic toxicity occurring in severe acute arsenic poisoning may include acute tubular necrosis with acute renal failure.
- Chronic renal insufficiency from cortical necrosis has also been reported.
- The precipitating cause of renal injury may be hypotensive shock, hemoglobinuric or myoglobinuric tubular injury, or direct effects of arsenic on tubule cells.
- Glomerular damage can result in proteinuria.
- The kidney is not the most sensitive target organ for chronic arsenic toxicity as other organ systems may be affected at lower doses.
- There is limited strength of association between chronic arsenic exposure and renal cancer [IARC 2004; NRC 2000].
- Arsine gas is more nephrotoxic than arsenic. However, both can cause acute tubular necrosis [Giberson et al. 1976].
Both acute and chronic exposure to high levels of arsenic may result in a wide range of adverse cardiovascular effects.
- There is limited strength of association between chronic arsenic exposure and peripheral vascular disease, hypertension, and cardiovascular disease [IARC 2004].
- Acute arsenic poisoning may cause both diffuse capillary leakage and cardiomyopathy, resulting in shock.
- The extent of cardiovascular injury may vary with
- age,
- arsenic dose, and
- individual susceptibility.
- In acute arsenic poisoning (such as suicide attempts), diffuse capillary leakage may lead to
- delayed cardiomyopathy,
- hypotension,
- shock,
- transudation of plasma, and
- vasodilation.
- Arsenic ingestion from contaminated beer has been reported to cause outbreaks of cardiomyopathy [Reynolds 1901; Aposhian 1989; Rosenman 2007].
- Other reports of arsenic poisoning have resulted in peripheral vascular disease, rather than congestive heart failure [Engel et al. 1994].
- Inhibition of endothelial nitric oxide synthase, changes in coagulation and inflammation due to arsenic exposure have been shown in experimental studies to contribute to atherosclerosis [Simeonova and Luster 2004].
- Hypertension has been reported with long-term exposure to arsenic [Chen et al. 1995].
- Ingesting arsenic levels of 0.8 to 1.82 ppm in drinking water (normal concentrations of arsenic in drinking water are lower than .01 ppm) as reported in Chile and Taiwan have resulted in an increased prevalence of peripheral vascular disease and cardiovascular mortality [Rosenman 2007].
- Myocardial damage can result in a variety of electrocardiographic findings, including
- broadening of the QRS complex,
- prolongation of the QT interval,
- ST depression,
- flattening of T waves, and
- atypical, multifocal ventricular tachycardia.
- Gangrene of the extremities, known as “blackfoot disease”, has been reported with drinking arsenic-contaminated well water in Taiwan, where the prevalence of the disease increased with increasing age and well water arsenic concentration (170–800 parts per billion) [ATSDR 2007].
- Pathologically, blackfoot disease was due to arteriosclerotic or thromboangiitis obliterans. After the water supply was changed, the vascular and cardiovascular mortality reversed [Pi et al. 2005; Chang et al. 2004].
- Persons with blackfoot disease also had a higher incidence of arsenic-induced skin cancers. However, investigators believe other vasoactive substances found in the water may have been contributory [ATSDR 2007].
- Vasospastic (Raynaud’s) disease in arsenic-exposed smelter workers and German vineyard workers has been reported. Smelter workers had a total exposure of 4 to 9 grams of arsenic, compared to the 20 grams of arsenic exposure reported for those with blackfoot disease [Rosenman 2007].
- Drinking arsenic-contaminated water in Chile was associated with an increase of vasospastic changes (Raynaud’s disease) and thickening of the small and medium sized arteries in autopsied children [Garcia-Vargas and Cebrian 1996].
- Arsenic ingestion affects the cardiovascular system, altering myocardial depolarization and causing cardiac arrhythmias and hypertension in some populations [Guha 2003].
- Inorganic arsenical pesticides are now generally not used by vineyard workers, and the organic arsenicals that are used have not been associated with vasospastic changes [Rosenman 2007].
- Increased cardiovascular mortality in occupationally exposed groups may be masked by the healthy worker effect [Hertz-Picciotto et al. 2000].
In studies that support an association, arsenic-exposed patients may develop destruction of axonal cylinders, leading to peripheral neuropathy. This has been reported at acute high doses (>2 milligram (mg) arsenic (As)/kilogram (kg)/day) as well as from repeated exposures to lower levels (.03 – 0.1 mg As/kg/day) [Chakraborti et al. 2003a, 2003b; ATSDR 2007].
- Arsenic may cause encephalopathy at acute high doses (> 2mg As/Kg/day) [Uede and Furukawa 2003; Vantroyen et al. 2004; ATSDR 2007].
Arsenic poisoning can cause peripheral neuropathy. The lesion is a sensory-motor axonopathy.
- The classic finding is a peripheral neuropathy involving sensory greater than motor neurons in a symmetrical, stocking glove distribution [Murphy et al. 1981].
- In high-level arsenic exposures, onset of neuropathy may occur after 7 to 14 days, with intense
- increased sweating in the distal lower extremities,
- muscle cramps,
- muscle tenderness,
- numbness,
- paresthesia, and
- spontaneous pain [Bleecker 2007].
- Sensory effects, particularly painful dysesthesia, occur earlier and may predominate in moderate poisoning, whereas ascending weakness and paralysis may be evident in more severe poisoning.
- Those cases may at first seem indistinguishable from Guillain-Barré syndrome (acute inflammatory demyelinating polyneuropathy) [Donofrio et al. 1987].
- Cranial nerves are rarely affected, even in severe poisoning.
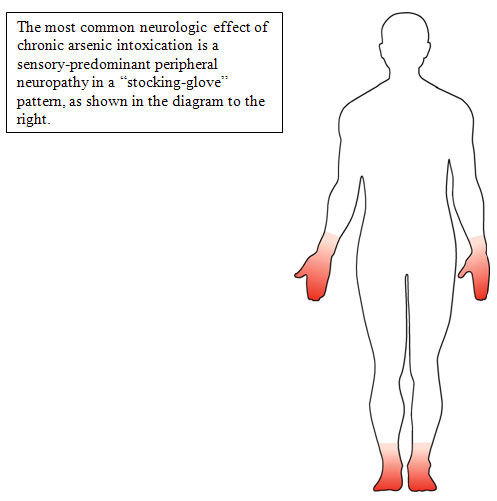
- The mechanism of arsenic neuropathy may be similar to the neuropathy of thiamine deficiency [Sexton and Gowdy 1963], whereby arsenic inhibits the conversion of pyruvate to acetyl coenzyme A and thus blocks the Krebs cycle.
- The neurotoxic forms of arsenic include inorganic trivalent (arsenite) and pentavalent (arsenate) and the methylated metabolites, monomethyl arsonic acid and dimethylarsenic acid [Foa et al. 1984].
Encephalopathy has been reported after both acute and chronic exposures.
- Onset may begin within 24 to 72 hours following acute poisoning, but it more often develops slowly as a result of chronic exposure [Beckett et al. 1986].
- The neuropathy is primarily sensory, with chronic exposure affecting vibration and positional sense to a greater extent than other modalities. Weakness of intrinsic muscles of the extremities is mild when present in chronic arsenic exposure [Bleecker 2007].
- The neuropathy is primarily due to destruction of axonal cylinders.
- Nerve conduction and electromyography studies most frequently show a sensory-motor axonopathy and can document severity and progression. A dose response effect has been reported between environmental exposure to arsenic-containing dust and vibrotactile threshold, tremor intensity, nerve conduction studies, and standing steadiness [Gerr and Letz 2000].
- Elevated vibration threshold has been associated with a cumulative arsenic index (drinking water arsenic exposure) and urinary arsenic levels [Hafeman et al. 2005].
- Subclinical neuropathy, defined by the presence of abnormal nerve conduction, but no clinical complaints or symptoms, has been described in chronically exposed individuals [Tseng 2003; ATSDR 2007].
- Recovery from neuropathy induced by chronic exposure to arsenic compounds is generally slow, sometimes taking years, and complete recovery may not occur.
- The prognosis for recovery in mild cases of neuropathy is excellent [Bleecker 2007].
- Follow-up studies of Japanese children who chronically consumed arsenic contaminated milk revealed an increased incidence of
- cognitive deficits,
- epilepsy,
- other brain damage, and
- severe hearing loss (ATSDR 2007).
- Hearing loss as a sequela of acute or chronic arsenic intoxication has not been confirmed by other case reports or epidemiologic studies [ATSDR 2007].
- There is limited strength of association between chronic arsenic exposure and neurologic effects, per the International Agency for Research on Cancer (IARC) and the National Research Council (NRC) [IARC 2004; NRC 2000].
Pigment changes and palmoplantar hyperkeratoses are characteristic of chronic arsenic exposure.
- Benign arsenical keratoses may progress to malignancy.
- Delayed effects of acute or chronic exposure may be seen as Mee’s lines in nails.
- Mees lines are horizontal lines in the nails of digits.
The skin lesions occurring most frequently in arsenic-exposed humans are
- hyperkeratosis,
- hyperpigmentation, and
- skin cancer.
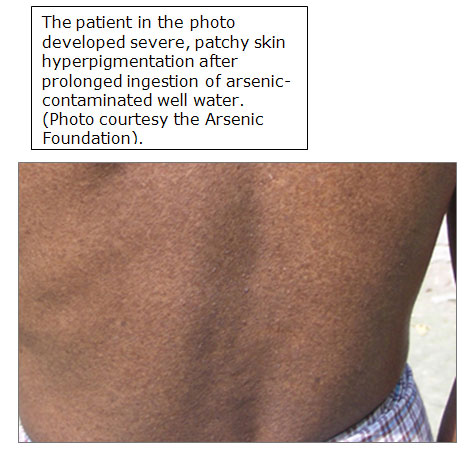
Patchy hyperpigmentation, a pathologic hallmark of chronic exposure, may be found anywhere on the body.
- Patchy hyperpigmentation occurs particularly on the
- axillae,
- eyelids,
- groin,
- neck,
- nipples, and
- temples.
- The common appearance of the dark brown patches with scattered pale spots is sometimes described as “raindrops on a dusty road”.
- In severe cases, the pigmentation extends broadly over the chest, back, and abdomen.
- Pigment changes have been observed in populations chronically consuming drinking water containing 400 ppb or more arsenic [ATSDR 2007]. Arsenical hyperkeratosis occurs most frequently on the palms and soles.
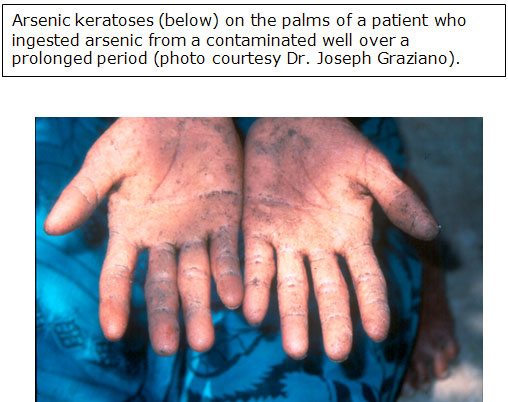
- Keratoses usually appear as small corn-like elevations, 0.4 to 1 centimeter (cm) in diameter.
- In most cases, arsenical keratoses show little cellular atypia and may remain morphologically benign for decades [ATSDR 2007].
- In other cases, cells develop marked atypia (precancerous) and appear indistinguishable from Bowen’s disease, which is an in situ squamous cell carcinoma discussed in Carcinogenic Effects later in this section [ATSDR 2007].
- Basal cell carcinomas have also been reported [Cohen and Moore 2007].
- Confounding factors for arsenic-induced skin cancer may include exposure to sunlight, chronic liver disease, and nutritional status [Hsueh et al. 1995].
Inhalation of high concentrations of arsenic compounds produces irritation of the respiratory mucosa.
- Smelter workers experiencing prolonged exposures to high concentrations of airborne arsenic at levels rarely found today had inflammatory and erosive lesions of the respiratory mucosa, including nasal septum perforation.
- Lung cancer has been associated with chronic arsenic exposure in smelter workers and pesticide workers [ATSDR 2007].
Bone marrow depression may result from acute or chronic arsenic intoxication and may initially manifest as pancytopenia.
- Both acute and chronic arsenic poisoning may affect the hematopoietic system.
- A reversible bone marrow depression with pancytopenia may occur.
- Anemia and leukopenia are common in chronic arsenic toxicity and are often accompanied by thrombocytopenia and mild eosinophilia.
- The anemia may be normocytic or macrocytic, and basophilic stippling may be noted on peripheral blood smears [Kyle and Pearse 1965; Selzer 1983].
- According to the NRC and IARC, there is a suggestive association between chronic arsenic exposure and immunosupression [NRC 2000; IARC 2004].
- Acute intoxication with arsine gas can cause fulminant intravascular hemolysis.
Increased frequency of spontaneous abortions and congenital malformations has been linked to arsenic exposure.
- Arsenic is a reproductive toxicant and a teratogen [Shalat 1996]. It is readily transferred across the placenta, and concentrations in cord blood are similar to those in maternal blood.
- A published case report described acute arsenic ingestion during the third trimester of pregnancy, leading to delivery of a live infant that died within 12 hours. Autopsy revealed intra alveolar hemorrhage and high levels of arsenic in the brain, liver, and kidneys [ATSDR 2007].
- A study of women working at or living near a copper smelter where ambient arsenic levels were elevated reported increased frequencies of spontaneous abortions and congenital malformations [Nordstrom et al. 1979].
- The frequency of all malformations was twice the expected rate and the frequency of multiple malformations was increased fivefold [Nordstrom et. al. 1979].
- However, a number of other chemicals, including lead, cadmium, and sulfur dioxide, were also present, and thus it is difficult to assess the role of arsenic in the etiology of these effects.
The carcinogenicity of arsenic in humans has been established.
| Agency | Carcinogenicity Classification | Description |
|---|---|---|
| International Agency for Research on Cancer | 1 | Known human carcinogen |
| National Toxicology Program | – | Known human carcinogen |
| U.S. Environmental Protection Agency | Group A | Known human carcinogen |
- In humans, chronic arsenic ingestion may cause cancers of the
- bladder,
- kidney,
- liver,
- lung,
- prostate, and
- skin (ATSDR 2007).
- Chronic inhalation of arsenicals has been associated with lung cancer and angiosarcoma (a rare form of liver cancer) has been reported [Falk et al. 1981].
- Several large-scale epidemiological studies of arsenic exposure have shown association and/or dose response trends for tumors of the
- bladder,
- kidney,
- liver,
- lung, and
- prostate [ATSDR 2007].
- According to IARC and NRC, the association between chronic arsenic exposure and cancer is strongest for skin, lung, and bladder cancer. Liver (angiosarcoma), kidney, and other cancers have limited strength of association [IARC 2004; NRC, 2000].
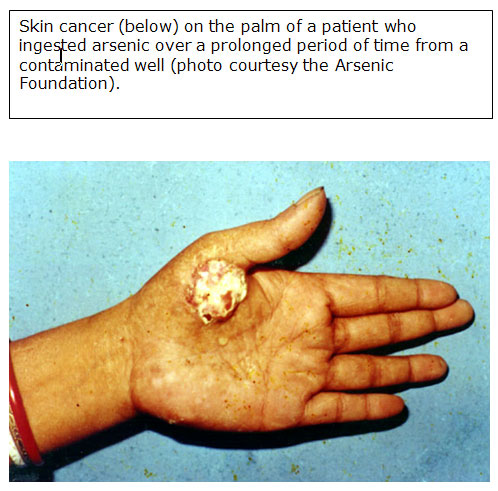
Latency for skin cancer associated with ingestion of arsenic may be 3 to 4 decades, while the noncarcinogenic skin effects typically develop several years after exposure [ATSDR 2007].
- An increased risk of skin cancer in humans is associated with chronic exposure to inorganic arsenic in contaminated water and the workplace.
- Arsenic-induced skin cancer is frequently characterized by lesions over the entire body, mostly in unexposed areas such as the
- palms,
- soles, and
- trunk.
More than one type of skin cancer may occur in a patient.
- Most of the Taiwanese who developed skin cancer in association with ingested arsenic-contaminated drinking water had multiple cancer types [ATSDR 2007]. The most commonly reported types, in order of decreasing frequency, were
- intraepidermal carcinomas (Bowen’s disease),
- squamous cell carcinomas, and
- basal cell carcinomas.
- Seventy-two percent of the Taiwanese with skin cancer also had hyperkeratosis, and 90% had hyperpigmentation.
- Some hyperkeratinized lesions can develop into intraepidermal carcinoma, which may ultimately become invasive. The lesions are sharply demarcated, round or irregular plaques that tend to enlarge; they may vary in size from 1 millimeter to more than 10 centimeters [ATSDR 2007].
- Arsenical basal cell carcinomas most often arise from normal tissue, are almost always multiple, and frequently occur on the trunk. The superficial spreading lesions are red, scaly, atrophic, and are often indistinguishable from Bowen’s disease by clinical examination.
- Arsenic-associated squamous cell carcinomas are distinguished from ultraviolet-induced squamous cell carcinomas by their tendency to occur on the extremities (especially palms and soles) and trunk, rather than on sun-exposed areas such as the head and neck. However, it may be difficult to distinguish other arsenic-induced skin lesions from those induced by other causes.
- Epidemiologic studies indicate that a dose response relationship exists between the level of arsenic in drinking water and the prevalence of skin cancers in the exposed population [ATSDR 2007].
- Excessive mortality rates due to arsenic-induced skin cancer have also been observed in vineyard workers with dermal and inhalation exposure [ATSDR 2007].
An association between lung cancer and occupational exposure to inorganic arsenic has been confirmed in several epidemiologic studies [Enterline et al. 1987], and arsenic is considered a cause of lung as well as skin cancer.
- In arsenic-exposed workers, there is a systematic gradient in lung cancer mortality rates, depending upon duration and intensity of exposure [ATSDR 2007].
- A higher risk of lung cancer was found among workers exposed predominantly to arsenic trioxide in smelters and to pentavalent arsenical pesticides in other settings.
- Neither concomitant exposure to sulfur dioxide nor to cigarette smoke was determined to be an essential co-factor in these studies.
There is suggestive [NRC 2000] or reasonably strong [IARC 2004] strength of association between chronic arsenic exposure and diabetes mellitus [NRC 2000; IARC 2004].
There is limited evidence of a diabetogenic effect from long-term arsenic exposure [Tseng et al. 2002].
Several studies have supported the notion of susceptibility to arsenic toxicity.
- Primary human hepatocytes have exhibited interindividual variations in the rate of arsenic methylation [Drobna et al. 2004].
- Arsenite uptake into the liver (and possibly other cells) may be increased in persons with poor nutritional status, which could overtax intrinsic detoxification mechanisms such as methylation [Rossman 2007].
- Increased uptake of arsenite into the liver of malnourished animals has been shown [Carbrey et al. 2003].
- Using urinary metabolites, differences in arsenic methylation capacity between population groups and individuals have been observed in several epidemiological studies [Hopenhayn-Rich et al. 1998; Chiou et al. 1995; Concha et al. 2002; Loffredo et al. 2003]. A low rate of arsenic methylation is related to a low rate of excretion.
- There is conflicting literature supporting the role of methylation in arsenic detoxification. Some literature suggests that methylation of inorganic arsenic may be a toxification rather than detoxification pathway [Kitchin 2001]. Other studies have suggested that other detoxifying mechanisms such as transport, antioxidant defenses, or resistance to apoptosis may be more important in protecting cells than methylation [Yoshida et al. 2004].
- Several studies have shown that arsenic-induced disease is increased in individuals who are undernourished or malnourished, possibly due to the decrease in arsenic methylation in such conditions [Hsueh et al. 1995; Mitra et al. 2004; Steinmaus et al. 2005].
- Animal studies have shown that arsenic methylation is affected by nutritional status. A diet low in protein, choline, or methionine showed reduced rates of arsenic excretion implying reduced rates of methylation [Rossman 2007].
- Methyl group donors such as folate might be important in arsenic detoxification in humans. A deficiency of folic acid promotes and supplementation of folate decreases the risk of cancers in rodents and humans [Rossman 2007]. Therefore, in cells that do not methylate arsenic, folate may be important for other reasons. Folate deficiency impairs DNA repair and alters the pattern of DNA methylation [Rossman 2007].
- Arsenic and selenium might be mutually antagonistic [Zeng et al. 2005].
- It has been hypothesized that low selenium levels in the soil may exacerbate arsenic toxicity and carcinogenicity in areas where there is high arsenic in the drinking water [Spallholz et al. 2004].
- In Taiwan, arsenic over exposed individuals had a reduction in the percentage of inorganic arsenic in urine while the percentage of DMA was increased with the concentration of urinary selenium and serum alpha tocopherol (vitamin E) [Rossman 2007].
- Since higher selenium levels in the body may promote the methylation of arsenic, it may be reasonable to suggest that a combination of selenium and Vitamin E or other antioxidants may be a safe (if taken as recommended) and possibly useful way to prevent adverse health effects in individuals with arsenic overexposure [Rossman 2007].
- Arsenic can cause serious effects of the neurologic, respiratory, hematologic, cardiovascular, gastrointestinal, and other systems.
- Arsenic is a carcinogen in multiple organ systems.
- Interindividual and population differences in arsenic methylation and nutritional status may be factors in susceptibility to arsenic toxicity.