What is Propylene Glycol?
Course: WB 4342
CE Original Date: March 20, 2020
CE Renewal Date: March 20, 2022
CE Expiration Date: March 20, 2024
Download Printer-Friendly version [PDF – 954 KB]
After completing this section, you will be able to
- describe the uses of propylene glycol, and
- explain the potential risk for propylene glycol toxicity.
Propylene glycol is a
- clear,
- colorless,
- viscous liquid with a faintly sweet taste.
Its chemical structure is CH3CH[OH]CH3OH.
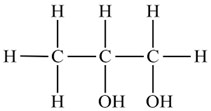
Propylene glycol and ethylene glycol have similar physical properties and uses. Their chemical structures differ by only one methyl group (ethylene glycol = HOCH2CH2OH; propylene glycol = CH3CH[OH]CH2OH).
Ethylene glycol is a potent cause of acute toxicity in humans. In contrast, propylene glycol is a “generally recognized as safe” additive for foods and medications.
Most reported cases of propylene glycol toxicity have resulted from propylene glycol used as a diluent for intravenous administration of benzodiazepines (Kraut and Kurtz 2008).
Synonyms for propylene glycol (ATSDR 1997) include
- 1,2-propanediol,
- 1,2-dihydroxypropane,
- methyl glycol, and
- trimethyl glycol.
Propylene glycol is generally recognized as safe by the Food and Drug Administration (FDA) (FDA 2017) for uses in
- food and tobacco products,
- pharmaceuticals, and
- cosmetics.
It has a wide range of other practical applications (ATSDR 2008), including use in
- deicers,
- coolants,
- antifreeze,
- heat transfer and hydraulic fluids,
- plasticizers, and
- other applications (smoke screen, smoke simulator, etc.).
In the general population, propylene glycol exposure occurs primarily through ingestion of food and medications and through skin contact with cosmetics or topical medications. Propylene glycol is used as a solvent in cosmetics and pharmaceuticals, in various
- oral,
- injectable, and
- topical formulations.
Propylene glycol is a diluent found in many intravenous and oral drugs, including
- phenytoin,
- diazepam, and
- lorazepam.
No adverse health effects are likely to occur from normal use of these products. However, heavy use of injectable medications with propylene glycol has caused excess levels of propylene glycol in the body (Horinek et al. 2009; Louis et al. 1967; Neale et al. 2005; Seay et al. 1997; Wilson et al. 2000; Yorgin et al. 1997; Zar et al. 2007; Zosel et al. 2010). Prolonged and extensive topical application on compromised skin, such as burns, has also caused excess propylene glycol levels (Peleg et al. 1998).
Patients in intensive care, for example, might experience toxicity from either of the following:
- Excessively large or rapidly infused intravenous injections of propylene glycol-containing medications (Horinek et al. 2009; Louis et al. 1967; Neale et al. 2005; Seay et al. 1997; Wilson et al. 2000; Yorgin et al. 1997; Zar et al. 2007; Zosel et al. 2010)
- Prolonged dermal contact during treatment of burns (Peleg et al. 1998)
Patients at risk for propylene glycol toxicity (Lim et al. 2014) include the following:
- Patients with underlying kidney disease
- Patients with less effective or impaired alcohol dehydrogenase enzyme systems (e.g., children younger than 4 years, pregnant women, patients with hepatic disease, and patients treated with disulfiram or metronidazole)
- Patients with epilepsy
- Burn patients who receive extensive dermal applications of propylene glycol
Absorption of propylene glycol from the gastrointestinal tract is rapid. The maximal plasma concentrations in humans occur within 1 hour after ingestion.
Metabolites
Propylene glycol is metabolized in the liver by alcohol dehydrogenase to
- lactic acid, and then
- pyruvic acid.
Both of these metabolites are normal constituents of the citric acid cycle and are further metabolized to
- carbon dioxide and
- water.
About 45% of an absorbed propylene glycol dose is excreted unchanged by the kidneys or as the glucuronide conjugate.
Half-life
In adults with normal liver and kidney function, the terminal half-life of propylene glycol ranges from 1.4 hours to 3.3 hours (Speth et al. 1987). In contrast, the mean half-life is significantly longer in infants — 19.3 hours (range: 10.8–30.5 hours) — because of decreased renal elimination (Lim et al. 2014).
Although propylene glycol is a commonly used solvent for intravenous medications, it might become toxic when administered in large doses over a short period (Bledsoe and Kramer 2008; Zar et al. 2007). Iatrogenic propylene glycol overdose can cause the following:
- Hyperosmolality and an anion gap metabolic acidosis, often accompanied by acute kidney injury, and potential multisystem organ failure (Arroliga et al. 2004; Greller and Gupta 2017; Tietze and Fuchs 2018; Wilson et al. 2000; Wilson et al. 2005; Yahwak et al. 2008; Zar et al. 2007)
- Refractory hypotension (Wilson et al. 2000)
- Arrhythmias (Louis et al. 1967)
- Hemolysis (Demey et al. 1988)
- Renal dysfunction (e.g., increased serum creatinine concentrations, proximal renal tubular cell injury, etc.) (Yaucher et al. 2003; Yorgin et al. 1997)
- Seizure, coma (Greller and Gupta 2017)
Pediatric patients also might develop CNS depression and seizures (Lim et al. 2014; O’Donnell et al. 2000).
Propylene glycol toxicity should be suspected in any patient receiving medication that contains propylene glycol as a diluent or solvent and who has
- hyperosmolality,
- lactic acidosis,
- acute kidney injury, or
- a clinical scenario similar to sepsis or systemic inflammatory response syndrome (SIRS) (Zar et al. 2007).
The clinical diagnosis of propylene glycol intoxication may be difficult because many hospitals do not measure propylene glycol levels. However, the osmolar gap, anion gap, and lactate are commonly elevated in propylene glycol intoxication (Lim et al. 2014).
An osmolar gap at 48 hours after continuous infusion strongly predicts propylene glycol accumulation. An elevated anion gap and lactic acidosis are poor indicators (Arroliga et al. 2004; Barnes et al. 2006; Wilson et al. 2005; Yahwak et al. 2008; Zar et al. 2007).
An osmolar gap >10 mmoles/L suggests that the serum propylene glycol concentration is high enough to cause toxicity (Barnes et al. 2006; Tietze and Fuchs 2018; Yahwak et al. 2008).
Because this disorder is iatrogenic, prevention by limiting the dosage of propylene glycol given to patients in the intensive care unit might be the best treatment [(Kraut and Kurtz 2008). Healthcare providers should consider a 50% reduction in the maximum daily dose for patients with underlying risk factors (see discussion on “Who’s at Risk”). The maximum daily dose of drug for a pediatric patient can be extrapolated from the adult data (based on a 70-kg patient) (Lim et al. 2014).
Metabolic acidosis caused by large amounts of propylene glycol in injected medications can be treated by discontinuing the offending medication and providing sodium bicarbonate and fomepizole (Zosel et al. 2010).
In severe cases, hemodialysis is effective in correcting hyperosmolality by removing propylene glycol from the blood (Demey et al. 1988; Kraut and Kurtz 2008; Lim et al. 2014; Parker et al. 2002; Wilson et al. 2000).
No workplace or environmental standards govern propylene glycol.
Propylene glycol is “generally recognized as safe” by the U.S. Food and Drug Administration (FDA) (FDA 2017). FDA considers an average daily dietary intake of 23 mg/kg of body weight to be safe for persons 2–65 years of age (ATSDR 2008).
- Various foods, cosmetics, and pharmaceutical products contain propylene glycol.
- Propylene glycol is metabolized to compounds that are normal constituents of the citric acid cycle.
- Propylene glycol toxicity generally is not a factor in environmental or occupational exposures.
- Iatrogenic propylene glycol overdose is the most common cause of propylene glycol poisoning.
- The major toxicological effects of propylene glycol poisoning include the following:
- Hyperosmolality
- Elevated lactate
- Refractory hypotension
- Arrhythmias
- Hemolysis
- Renal dysfunction
- Because this disorder is iatrogenic, prevention by limiting the dosage of propylene glycol given to patients in the intensive care unit might be the best treatment.
Quiz 18: To review relevant content, see “Uses” in this section.
Quiz 19: To review relevant content, see “Biological Fate” in this section.
Quiz 20: To review relevant content, see “Biological Fate” in this section.