What Is the Biologic Fate of Carbon Tetrachloride in the Body?
CCl4 is well absorbed from the respiratory and gastrointestinal tracts. Dermal absorption of liquid is possible, but dermal absorption of the vapor is limited. Absorbed CCl4 is distributed throughout the whole body, with highest concentrations in liver, brain, kidney, muscle, fat, and blood.
CCl4 is primarily metabolized in the liver, where it is transformed by cytochrome P450 enzymes to a toxic metabolite.
Any unchanged parent compound is eliminated primarily in exhaled air.
Pulmonary: Absorption from the lung was estimated to be about 60% in humans [Lehmann and Schmidt-Kehl 1936; Astrand 1975].
Gastrointestinal: Results from several animal studies have shown that CCl4 is rapidly and extensively absorbed from the gastrointestinal tract [Paul et al. 1963; Kim et al. 1990].
Dermal: Liquid CCl4 is readily absorbed through the skin of humans. A study showed that immersion of both hands in liquid CCl4 for 30 minutes would yield an exposure equivalent to breathing 100-500 ppm for 30 minutes [Stewart and Dodd 1964].
CCl4 is distributed to all organs [Bergman 1983; Paustenbach et al. 1986], and concentrates in the
- Fat,
- Liver,
- Bone marrow,
- Adrenals,
- Blood,
- Brain,
- Spinal cord, and
- Kidney.
Carbon tetrachloride is metabolized primarily in the liver.
The first step in metabolism is a phase I dehalogenation reaction, which converts CCl4 to the trichloromethyl radical (CCl3). This reaction is governed primarily by the cytochrome P450 isoform CYP2E1, though additional isoforms such as CYP3A4 can also metabolize CCl4 when it is present in high concentrations [Slater 1984; Slater et al. 1985; Recknagel et al. 1989; Weber et al. 2003]. The centrilobular localization of CYP2E1 in the liver explains the histopathological findings of CCl4-induced liver damage, which will be discussed in the following section (“What Are the Toxicological Effects of Carbon Tetrachloride?”).
The CCl3 radical can have several different fates (see Figure 2). It might
- React with intracellular molecules to cause lipid peroxidation and other forms of oxidative damage,
- Be converted to the trichloromethyl peroxy radical, OOCCl3 [Weber et al. 2003],
- Acquire a hydrogen atom to form chloroform (CHCl3),
- Combine with other CCl3 radicals to form hexachloroethane (Cl3CCCl3), or
- Be further metabolized to carbon dioxide (CO2) by successive oxidation reactions.
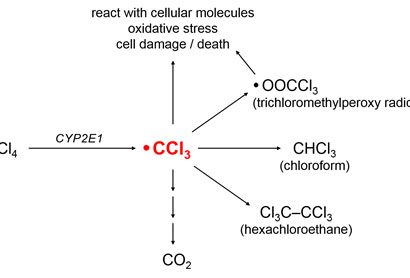
Figure 2.Common metabolic fates of CCl3.
One animal model estimates that 60% of an inhaled dose of CCl4 is metabolized, and the remaining 40% is excreted unchanged. Approximately 96% of this metabolized CCl4 generates free radicals, as described above; the remaining 4% is ultimately converted to CO2 [Paustenbach et al. 1988].
Animal studies evaluated elimination of carbon tetrachloride following oral or inhalation exposures. In rats receiving equivalent doses by inhalation or bolus gavage, terminal elimination half-lives (t1/2) were about 4 hours [EPA 2010].
In humans and animals exposed to carbon tetrachloride by any route, most of the unmetabolized parent compound is excreted in exhaled air. Animal studies show that volatile metabolites (such as chloroform [CHCl3]) are released in exhaled air, whereas its nonvolatile metabolites are excreted in feces and, to a lesser degree, in urine [EPA 2010].